Mini Lesson/Activity
Aurora Bracelet
Overview
Use art to demonstrate your knowledge of aurora.
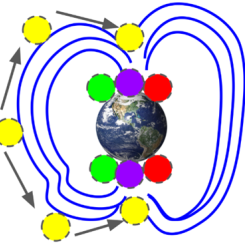
The aurora bracelet, or keychain, uses red, green, and purple pony beads, arranged with an “O” bead for Oxygen and an “N” bead for Nitrogen to remind learners of the gasses in the atmosphere that get excited to create the colors of an aurora.
Materials Needed
- Make an Aurora Bracelet Handout
- String (about one yard)
- Pony beads
- 2 red pony beads
- 4 green pony beads
- 2 purple pony beads
- Letter beads
- 1 “O” letter bead
- 1 “N” letter bead
- scissors
Student Directions
Remember to never look directly at the Sun without proper safety equipment.
Context:
When the solar wind interacts with gases in the atmosphere, ionization occurs, causing the gases in the ionosphere to glow.
This is similar to how the neon lights that you see in store windows work. Electricity excites the different gases in the neon light tubes; different gases glow different colors.
The Earth’s atmosphere is made up of 78% nitrogen, 21% oxygen, and a small amount of argon, carbon dioxide, methane, and some other trace amounts of gases.
When oxygen atoms and molecules collide, they emit the visible green and red light of an aurora. When nitrogen atoms and molecules collide, they emit the visible purple and blue light of an aurora.
Create:
To remember what the colors mean, we can make an aurora bracelet. If you don’t wear bracelets, you can attach it to a keychain or hang the beads in a window!
Use the Make an Aurora Bracelet Handout for directions on how to make the bracelet.
You will string the beads in order, which will start with the upper atmosphere and move down toward the lower atmosphere.
- String the two red beads onto the yarn first; red is caused by energized oxygen high in the atmosphere.
- Next, string the “O” letter bead for oxygen. The “O” will be between the red and green beads, both colors created by oxygen.
- Next, string the four green beads. Green is created by oxygen in the middle atmosphere, and we use four beads to remind us that it is the most common color seen in aurora.
- Next, string the “N” letter bead for nitrogen.
- String the two purple beads last; purple is caused by energized nitrogen in the lower atmosphere.
Teacher Note
Teachers, these mini lessons/student activities are perfect "warm up" tasks that can be used as a hook, bell ringer, exit slip, etc. They take less than a class period to complete. Learn more on the "My NASA Data What are Mini Lessons?" page.
Teachers who are interested in receiving the answer key, please complete the Teacher Key Request and Verification Form. We verify that requestors are teachers prior to sending access to the answer keys as we’ve had many students try to pass as teachers to gain access.

Disciplinary Core Ideas:
- PS2B: Types of Interactions
- PS4A: Wave Properties
- PS4B: Electromagnetic Radiation
- ESS1A: The Universe and its Stars
Crosscutting Concepts:
- Patterns
- Cause and Effect
- Structure and Function
Science and Engineering Practices:
- Developing and Using Models