Lesson Plans
Heating Things Up
Overview
Working in small groups, students will explore the energy transfer of different earth materials when they are heated up and cooled down. The students graph the changes in temperatures that occur over a 15-minute period of heating the earth material up and a 15-minute period of the earth material cooling down. Credit: Dr. Kevin Czajkowski & Janet Struble, University of Toledo
Materials Required
- 5 plastic containers of the same size
- Hand-Held Infrared Thermometer (IRT) or 5 thermometers
- Sand
- Soil
- Grass
- Water
- Gravel or Rocks
- Additional Materials that could be substituted or used as an extension to the current materials: light mulch, dark mulch, colored sand, and artificial turf
- A sunny day or 5 desk lamps or clip-on shop lights with 100-watt light bulbs
- Stopwatch or timer
Preparation
Put equal amounts of each earth material in separate containers.
Procedure
Engage
- Ask students to think about a hot sunny day at the beach when they are barefoot.
- What surfaces would you walk on and why? Why are some surfaces cool to your touch? Why are some hot to your touch? From where did the hot surfaces get their energy? [Sun]
Explore
- Tell the students that today they are going to investigate what happens to air and different earth materials when they are exposed to the Sun. Mention some of the materials the students may have told you earlier such as sand, grass, rocks, etc. Try to get the students to mention water, bare soil, and air.
- Each group will record the temperature change of each earth material. Each container should have about the same amount of material. If it is a sunny day, this activity can be done outside. If not, desk lamps or shop lights can be used to simulate the Sun’s energy.
- If you are using thermometers, place them in the earth material.
- For each earth material, designate a recorder that will record the temperatures and person that will measure the temperature in degrees Celsius.
- Record the beginning temperature of each material. Start the timer. The timekeeper will tell the students when they should measure the temperature with the IRT or read the thermometers. If you are using one IRT, try to have the students take the measurements as close together in time as possible.
- Students will record the temperature of the earth material in the Sun or under the light every 3 minutes for a total of 15 minutes. After 15 minutes, the earth materials are shaded or the lights are turned off. Measure the temperature every 3 minutes for the next 15 minutes. The total elapsed time is 30 minutes with 11 temperature measurements.
- See the example of a data table that students can fill in or have the students create the data table in their science notebooks.
- Each group shares their data either by writing the numbers on a large chart or on an overhead transparency. Each student records the data on Data Table Sheet or in one’s notebook.
Students should now review the data.
- Does each material in the container start at the same temperature? [No]
- How can we compare them to see which one had the most change in temperature? [Subtract the current temperature from the initial temperature and you will calculate the change in the temperature.]
- Using these data, encourage students to observe the change in the material over time. Emphasize: subtract the temperature from the beginning temperature. This means the first row in Data Table Sheet 2 will be all zeroes. Students fill in Data Table Sheet 2. This can be assigned for homework.
- The next day the teacher reviews what was done yesterday and brings out the Data Table Sheet 2. As a class, fill in the changes in temperature.
- One way scientists analyze their data is by graphing the data. In order to look at what is happening with each material, you will graph the data of all materials on one graph. Each material's data will be graphed in a different color. Use colored pencils to identify the different materials. The teacher may want to assign the colors, such as blue will represent the water, green the grass, and so on.
- If needed, review how to do a graph. The x-axis displays the independent variable which is time; the y-axis displays the dependent variable – change in temperature. The students select appropriate and uniform intervals and write them on the graph paper.
- As students work on graphing the data, the teacher looks over the groups to make sure students are graphing correctly. If the teacher chooses this graphing exercise as an assessment, a rubric is provided at the end of this activity.
Explain
1. The following questions can be used for class discussion or as an individual assignment. Questions for discussion:
- Did all the earth materials heat up at the same rate? Explain the evidence for your answer.
- Did all the earth materials cool down at the same rate? Explain the evidence for your answer.
- Which earth material heated up the fastest? How do you know this? Explain why this may have happened.
- Which earth material cooled down the fastest? How do you know this? Explain why this may have happened.
- Did each earth material receive the same amount of energy? How do you know?
2. Explain that students should find from this investigation that water is the slowest to heat up and cool down. All the materials received the same amount of energy. So why do you think that the water increased so little? For water, it takes five times more heat energy to raise the temperature of an equal amount of dry soil or sand one degree. When the same amount of energy is absorbed equally by all of the earth materials, the temperature of the solid earth materials will increase faster and greater than water. In addition, water is a liquid that mixes. The energy for water is mixed throughout its volume while only the very surface of solids is heated.
3. Introduce the concept of radiation in the Earth’s atmosphere. The teacher may ask, “The Sun heated up each earth material in the containers. How does this happen? How does the energy from the Sun get from the Sun to the sand? Water? Grass? Soil? Rocks?” The teacher needs to draw out the students’ understanding of this first, then provide an explanation that will clear up misconceptions. This information is in the section “Background.” The following is a short synopsis.
- The energy that comes from the Sun is radiant energy that travels in waves through space. This energy makes up the electromagnetic spectrum. Some waves that you may be familiar with are visible light (which we can see), and radio waves (listening to radio station in your car). When the energy hits a molecule or atom such as molecules of sand or water, the molecule gains energy. This gain in energy makes the molecule vibrate or move faster. We say the molecule absorbed the energy. This molecular movement is what we call heat. The more the molecules move, the more heat is produced.
Disciplinary Core Ideas:
- PS3B: Conservation of Energy and Energy Transfer
- ESS3C: Human Impacts on Earth Systems
Crosscutting Concepts:
- Cause and Effect
- Scale, Proportion, and Quantity
Science and Engineering Practices:
- Planning and Carrying out Investigations
- Analyzing and Interpreting Data
The student will:
- perform an investigation that tests energy transfer of different Earth materials
- analyze and graph data
- infer what happens in the Earth’s atmosphere
- Why do different materials experience differences in surface temperature?
What is heat?
Temperature is defined as the average kinetic energy (energy of motion/vibration) of all the atoms or molecules in an object. The more kinetic energy the atoms and molecules in matter possess, the faster the molecules vibrate, and the hotter the matter will be. Whenever two materials of different temperatures are next to one another, thermal energy flows from an object of one temperature to an object of another temperature; this flowing energy is referred to as "heat". The kinetic energy of heat is associated with this flow of thermal energy that arises from temperature differences between different materials. All materials above absolute zero (-273.15° C) are able to transfer heat to colder materials.
What happens to this energy in materials?
How atoms and molecules move when they possess this internal kinetic energy depends on the state of matter of the material that contains the atoms and molecules. The three states of matter are solids, liquids and gases. In a solid material, the atoms and molecules are set in place but can rotate and vibrate. Therefore the temperature of the solid indicates the average rotational and vibrational energy. An example you can tell students is to stay seated in their seats and they cannot move their seats from their spot. Now the students can move their bodies while they are seated (vibrational) and they can turn their seats around (rotational) if they have wheels underneath them. As the solid absorbs energy, the molecules gain enough energy to move from their places and can slide past each other. The solid now changes to a liquid. In our classroom analogy, students possess enough energy to move from their spot, but they need to stay within the walls of the classroom and close to the other students. If the liquid absorbs even more energy, it turns into a gas. The atoms and molecules now possess so much energy that they can move in all directions, vibrate faster, and rotate faster. . Now relating this back to students, the students are spread out on the playground or gym. They can run in any direction, spin around, and wave their arms and hands. If the student happens to bump into another student or wall, the student just bounces off and changes direction.
In what ways does energy move?
Energy can move from one place to another by radiation, conduction, or convection.
Radiation
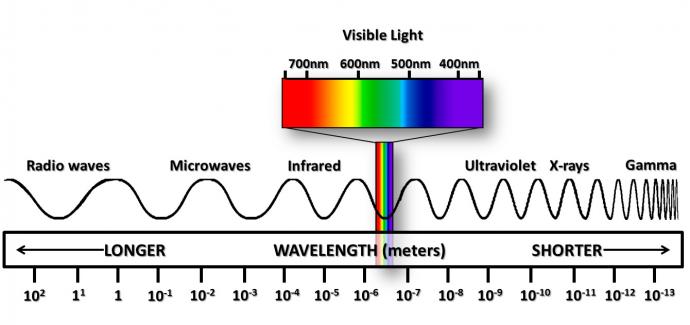
The Sun is our source of radiant energy. Inside the Sun, thermonuclear reactions are taking place. The result of these reactions is tons of energy! This energy travels through space in all directions in the form of electromagnetic radiation. The electromagnetic radiation constitutes the electromagnetic spectrum. This electromagnetic spectrum is comprised of waves of different wavelengths and energy levels. The visible spectrum of light is just one narrow band in this. The electromagnetic spectrum spans from radio waves having the longest wavelength and the least amount of energy to gamma rays having the shortest wavelength and the highest amount of energy.
Radiant energy travels through space. When it reaches an atom or molecule, the energy can do one of two things: a) the energy can be reflected in a different direction or b) the energy can be absorbed by an atom or molecule. The absorption of the energy increases the kinetic energy of the atom or molecule that, in turn, increases the temperature of the material, no matter if it is a solid, liquid, or gas.
As stated earlier, the Sun sends out all types of waves in the electromagnetic spectrum, but it sends the most waves in the visible part of the electromagnetic spectrum.
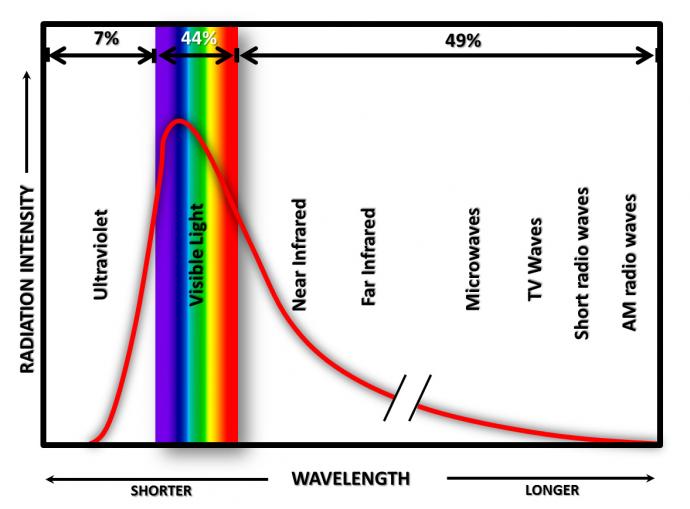
Most of the incoming visible radiation from the Sun reaches the surface. Air in the Earth’s atmosphere is mostly composed of nitrogen (78%) and oxygen (20%) molecules. These molecules do not absorb the Sun’s radiation in the visible part of the spectrum, but scatter the radiation or let it reach the surface. The characteristic scattering by these molecules is why the sky is blue. Radiation that the atmosphere lets through strikes the Earth's surface, and the energy is absorbed by atoms and molecules at the surface. The temperature of the surface then increases. This is the way that the Sun warms the surface of the Earth.
If the Sun is constantly shining on the Earth, why doesn’t the Earth keep getting warmer and warmer? The reason is that all matter, including the Earth’s surface, radiates energy. The Earth’s surface radiates energy towards the atmosphere in the form of infrared waves. As with the Sun’s radiant energy, some of the infrared waves pass through the atmosphere unchanged and make it to space, transferring energy away from Earth. Some of the infrared waves are absorbed by molecules in the atmosphere. Certain molecules in the air, such as water vapor and carbon dioxide, absorb the infrared radiation; this absorption increases their kinetic energy. As a result, the lower part of the atmosphere is heated more from below (infrared radiation from the Earth’s surface) than from above (radiation from Sun).
In the Earth system, the energy added from visible radiation from the Sun being absorbed at the surface and the energy transferred away from Earth to space by infrared radiation are in balance. In other words, the amount of radiant energy that reaches the Earth’s surface is equal to the amount of energy radiated back to space; therefore, the average net kinetic energy (average temperature) of Earth’s surface and atmosphere stays almost constant.
Conduction
Conduction is energy transfer by contact. When one molecule collides with another molecule, energy is transferred from the more energized molecule to the less energized molecule. As this continues to happen, the kinetic energy of the substance is increased, it gets hotter. The opposite also happens; a molecule can lose energy.
A small portion of the heating of Earth’s atmosphere occurs as a result of energy transferred to air molecules that have made contact with heated surfaces such as land and water. , In some situations, the Earth’s atmosphere can cool as a result of energy transferred by contact between air molecules and cold surfaces, such as when warm air moves over a cold body of water.
Convection
Let’s continue to look at the heated air in the Earth’s atmosphere. When air comes in contact with the heated surface of the Earth, energy is transferred to the air by conduction as mentioned previously. The kinetic energy of the land is transferred to the air molecules; the air molecules have more kinetic energy and move farther apart (gas expands). The increase in volume without an increase in the number of molecules makes air less dense. The lighter air (less dense) begins to rise. As it rises, it travels to higher levels in the atmosphere where the temperature is cooler. The molecules cool and lose their kinetic energy to the surroundings; the gas becomes denser and sinks down to the Earth’s surface. When the cool air comes in contact with the Earth’s surface, it heats up and the process starts again. This cycle of rising and falling of the air is convection. Convection has the net effect of transferring heat from the warmer parts of the atmosphere near the surface to cooler parts of the atmosphere at higher levels.
Convection can only happen in fluids, meaning liquids and gases. The molecules need to be able to move which cannot happen in a solid. Convection is usually thought of as the third way that energy is transferred in the atmosphere. A simple description is energy transportation. The processes by which energy transfers from one molecule to another are radiation and conduction. Convection is analogous to an elevator that carries heated (high energy) molecules up and cooled (lower energy) molecules down.
Teacher Preparation
Heat is a hard concept for students to understand. It is not a substance, but a form of energy. So, what does the temperature tell us about the amount of heat in the substance? Nothing! The thermometer does not measure the amount of heat; it measures the level of kinetic energy. This distinction is hard for students and even adults to understand. The big idea of this activity is that heat is energy; energy has many forms; energy can move from one place to another by radiation, conduction, or convection. One form of energy is the motion of molecules (atoms)—kinetic energy. Thermometers are used to detect this heat-transfer phenomenon; temperature is used to compare the kinetic energy of different substances as heat moves.
- Ability to observe and collect temperature data using thermometer
- Graphing skills
The following list is taken from Operation Physics Elementary / middle school physics education outreach project of the American Institute of Physics.
The web page Children’s Misconceptions about Science provides a list of misconceptions in several areas of physical science, including heat and temperature. Here are a few that you might hear in your own classroom:
STUDENTS MAY THINK… INSTEAD OF THINKING… Heat is a substance. Heat is not energy. Heat is energy. Temperature is a property of a particular material or object. (For example, students may believe that metal is naturally cooler than plastic.) Temperature is not a property of materials or objects. Objects exposed to the same ambient conditions will have the same temperature. The temperature of an object depends on its size. Temperature does not depend on size. Heat and cold are different. Cold is the absence of heat. Heat and cold can be thought of as opposite ends of a continuum. Cold is transferred from one object to another. Heat is transferred from one object to another. Heat moves from the warmer object to the cooler object. Objects that keep things warm (sweaters, mittens, blankets) are sources of heat. Objects keep things warm by trapping heat. Some substances (flour, sugar, air) cannot heat up. All substances heat up, although some gain heat more easily than others. Objects that readily become warm (conductors of heat) do not readily become cold. Conductors gain (and lose) heat easily.
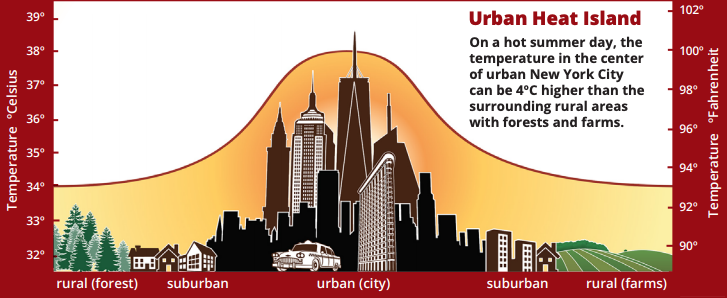
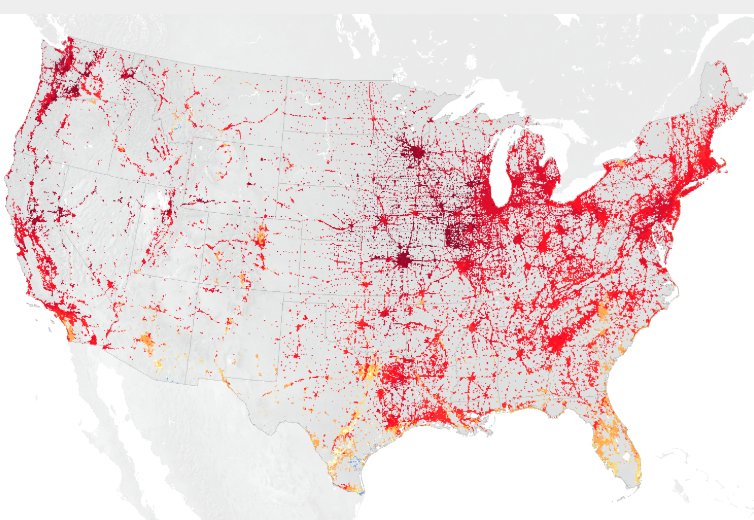
An urban heat island occurs when a city experiences much warmer temperatures than in nearby rural areas. Cities are full of rocky surfaces – asphalt, brick, and concrete – which increases the amount of energy from solar radiation they absorb. Urban areas often see temperatures rise 6°C (10°F) hotter than the surrounding suburbs and rural areas. These higher temperatures can cause people to become dehydrated or suffer from heat exhaustion. NASA analyzes surface temperature data from around the world to better understand the characteristics of cities that drive the development of urban heat islands.
- Atmospheric and Space Scientists – Investigate weather and climate-related phenomena to prepare weather and climate-related phenomena to prepare weather reports and forecasts for the public
- Computer and Information Scientists – Conduct research in the field of computer and information science
- Applications Software Developers – Develop and modify computer applications software that is used to communicate with satellites and people using satellite data
- Computer Programmers
- Systems Engineers
- Software Engineers
- Standalone Lesson (no technology required)