Lesson Plans
Energy Absorption on the School Yard (Lab Activity)
Overview
Students collect and analyze temperature data to explore what governs how much energy is reflected. This lab activity is designed to demonstrate that the albedo of a land surface can have a measurable impact on the surface temperature. This is a key aspect to begin developing an understanding of the absorption of energy at the surface of the Earth. (The albedo is the ratio of the reflected solar energy to the total incident solar energy—in other words, the fraction of solar energy reflected by a surface.) This lesson is part of a series, originally published as problem-based learning modules centered on the theme of energy in the Earth system that demonstrates the importance of surface type in the absorption of solar energy, and the importance of surface moisture in the Earth’s overall energy balance and, therefore, in determining temperature.
Materials Required
Version 1: Open Inquiry Method
- Thermometers (one per cup) or an infrared thermometer for each group to use with all cups
- Note: for information on how to use the infrared thermometer, visit the GLOBE Protocol for Surface Temperature
- paper cups (each with a hole in the bottom)
- lab notebook (or Student Sheets)
- clipboard
- pencil
- timer
- a nice sunny day (in UNSHADED areas)
Version 2: Guided Inquiry Method
- White and black construction paper (and other colors) (one of each per group)
- Thermometers for each piece of paper or one infrared thermometer for each group
- Note: for information on how to use the infrared thermometer, visit the GLOBE Protocol for Surface Temperature
- lab notebook (or Student Sheets)
- clipboard
- pencil
- timer
- a nice sunny day (in UNSHADED areas)
Procedure
Set the Stage and Address Prior Knowledge
- Present the Essential Question: Does the type of the ground surface influence its temperature? Provide time for students to generate thoughts, ideas, and additional questions about this topic.
- Think about the different types of surfaces that are outside on your campus, both natural and human-made. List them in your lab notebook. Make a claim as to whether you believe the surfaces will all be the same temperature. Explain why or why not.
- Now introduce the lab investigation. This lab can be done in a variety of manners, with respect to student inquiry. Its original intent is to have students design their own investigation.
Investigate
Version 1: Open-Inquiry Method
In this format, educators should provide the following to students, along with time to plan and execute the steps safely and accurately.
- Review the following key tasks and identify your investigation plan.
- How will you test your hypothesis?
- What surfaces will you measure?
- How will you use the materials provided?
- How will you collect data? record the data?
- What variables will you hold constant?
- How will you know if you are successful?
- Remind students of materials safety. Allow time to plan and execute safely.
- Students should record all steps and results in their notebook or Student Sheets.
Version 2: Guided-Inquiry Method
To scaffold this activity in a more guided-inquiry manner, see the following instructions:
- Review the investigation and distribute the Investigation's Student Sheets. Review any key vocabulary as needed.
- Describe the following (general) investigation plan with your students before going outside to execute the investigation.
- Identify the (safe) investigation area/s of the campus/playground. Do not put in a shaded area (or create a shadow where your thermometer is located.)
- Students will place large squares of different colors (e.g., construction paper).
- Gently lay the thermometers on the paper.
- Monitor the thermometers and collect data at 0, 2, 4, etc. minutes.
- Review the following key tasks and identify/describe to your students the investigation plan. Students should document this in their student sheets.
- How will you test your hypothesis?
- What surfaces will you measure?
- How will you use the materials provided?
- How will you collect data? record the data?
- What variables will you hold constant?
- How will you know if you are successful?
- Head outside with students and allow students to set up the investigation.
- After a set time (i.e., 0 min, 2 min, 4 min, etc.), examine the temperature differences between the materials. Collect these data.
Analyze and Interpret Data
- Return to class and allow students to analyze and graph data. The students may continue to work in teams on the completion of their Student Sheets.
Make Sense of the Data
- Students to complete the remainder of their student sheet.
- Discuss with the class
Closure
Visit Analyzing Surface Temperature Differences: Student Activity. Have students complete the mini-lesson as an Exit Slip.
Disciplinary Core Ideas:
- PS4B: Electromagnetic Radiation
- ESS2A: Earth Materials and Systems
Crosscutting Concepts:
- Cause and Effect
Science and Engineering Practices:
- Asking Questions and Defining Problems
- Planning and Carrying out Investigations
- Analyzing and Interpreting Data
- Engaging in Argument from Evidence
- Students will use and apply the practices of science.
- Students will define albedo and explain how it is determined
- Students will explain the effects of the type of ground surface on albedo and Earth's surface temperatures.
Does the type of the ground surface influence its temperature?
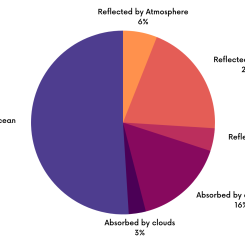
The Earth’s energy budget plays a major role in weather and climate around the world. Several key facts are evident if we follow the flow of energy from the Sun through the Earth system. Energy, like sound, travels as invisible waves of different sizes. We start by assigning an arbitrary measure of 100 units as the amount of solar energy received at the top of our atmosphere. The atmosphere and its elements (clouds, particles, molecules) reflect about 26 units back out to space and absorb another 19 units. The remainder of the energy passes through the atmosphere, with much of it (51 units) being absorbed by the Earth’s surface. Essentially, the energy absorbed by the land and the oceans is what drives atmospheric and oceanic circulations. Finally, about 4 units of the energy are reflected by the surface. The albedo, or reflectivity, of a surface, is the ratio of the reflected solar energy to the total incident solar energy—in other words, the measure of the fraction of solar energy reflected by a surface. The albedos of natural surfaces range from as low as .07 (93% of the energy is absorbed) in tropical forests and oceans with the Sun directly overhead, to .85 (only 15% of the energy is absorbed) for a fresh snow or ice surface at high latitudes.
Virtually all of the energy that heats the Earth’s surface is then transferred to the atmosphere and to space by several different mechanisms. First, all surfaces radiate (give off ) energy back through the atmosphere toward space. Also, heating from the Earth’s surface causes upward movement of the air above (convection) and changes the state of water from liquid to vapor form (evaporation). Convection, evaporation, and radiation from the surface exceed the total amount of energy that was absorbed by the surface to begin with! This is impossible unless there is a missing element of the energy budget. In fact, there is: the Earth’s atmosphere contains water vapor, carbon dioxide, and other greenhouse gases, which absorb energy radiated toward space and then emit some to space and some back to the Earth’s surface. Greenhouse gases are responsible for keeping the Earth’s temperature warm enough to support life as we know it. The exercises presented in the following pages are intended to explore what governs the amount of energy absorbed by the Earth’s surface and the important role of water in the Earth’s energy budget.
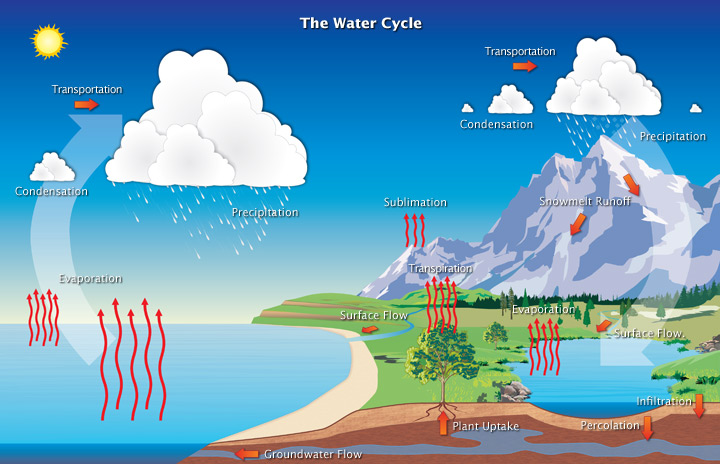
Following is a brief summary of the water cycle, also called the hydrological cycle:
- The heat energy required for evaporation, sublimation, or transpiration of water (the conversion of liquid water or ice on the Earth’s surface to gaseous water vapor in the air) - is stored in the vapor as latent heat. (A good example of latent heat is when someone comes out of a swimming pool. Energy is required to evaporate the water on the skin. This energy is taken from the surrounding skin, producing cooling, and stored in the water vapor.)
- As the moisture-laden air cools, some of the water vapor condenses back into cloud droplets (water vapor changes to liquid water), releasing the latent heat.
- Precipitation is absorbed by or accumulates on the Earth’s surfaces, infiltrates into the ground, or runs off into lakes, streams, and rivers, and then back to the seas.
- When enough cloud droplets grow to precipitable size, they fall to the Earth’s surface as rain/snow/ sleet, etc.
- The cycle begins to repeat itself as moisture from the Earth’s surfaces (oceans and land) evaporates, sublimates, or transpires again. Note that when precipitation occurs, a convergence (coming together) of moisture is required to sustain the precipitation process, concentrating the latent heating in the column of precipitating atmosphere. The water cycle’s redistribution of heat energy in the atmosphere not only cools the Earth’s surfaces, but it also produces circulations in the atmosphere. These circulations are significantly different than those that result from the uneven heating of the Earth’s surface by the Sun.
Water is a key element of the Earth’s energy balance. The Sun’s energy drives the water cycle, and in turn, water is a major factor in governing the surface temperature of the Earth.
- Standalone Lesson (no technology required)