Energy and Matter Cycles
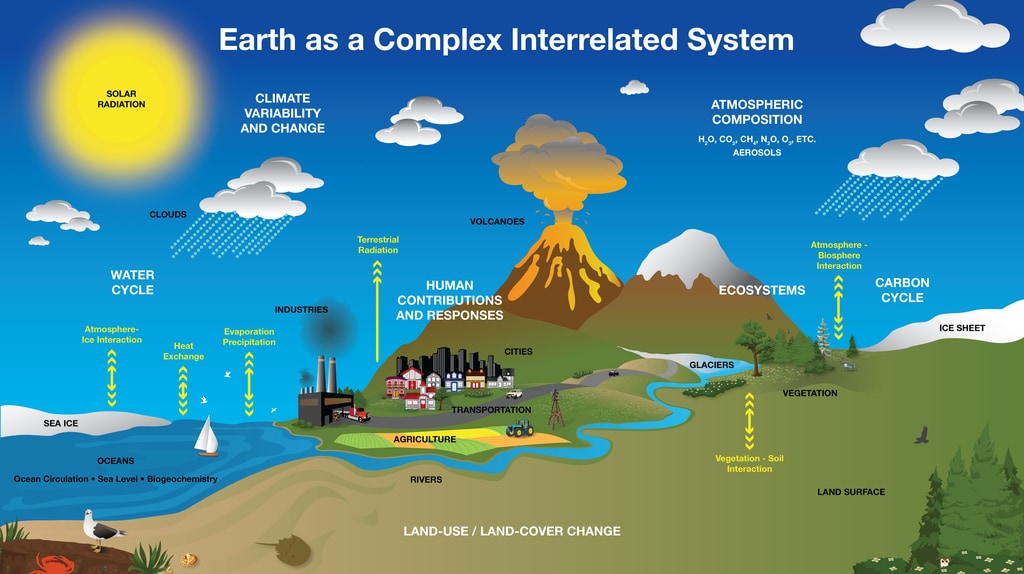
Diagram showing parts of the Earth system. Image Credit: NASA's Goddard Space Flight Center.
Explore the energy and matter cycles found within the Earth System.
Energy Cycle
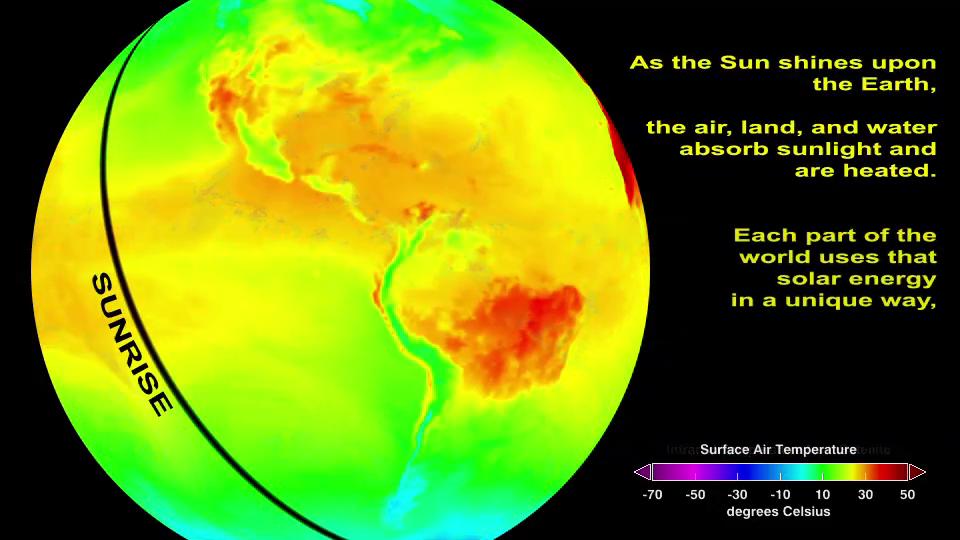
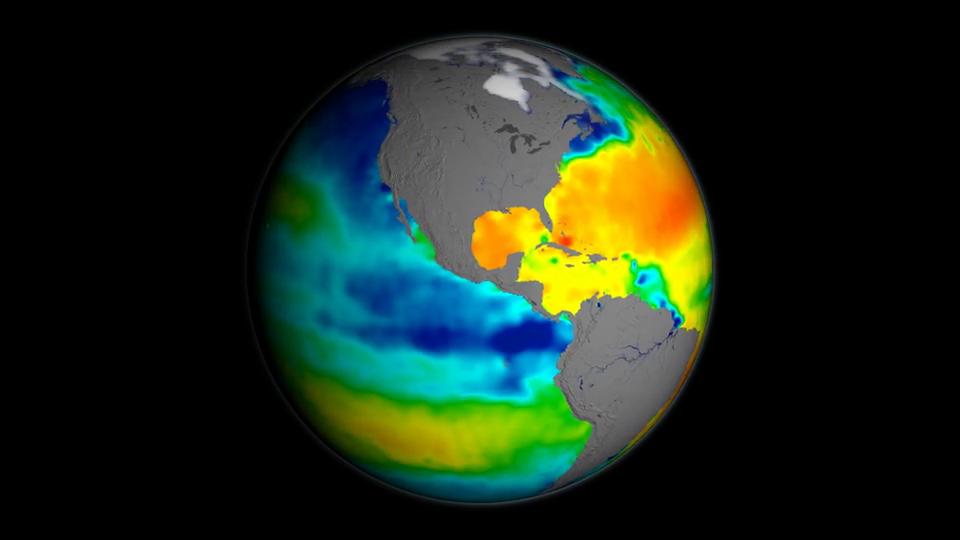
Energy from the Sun is the driver of many Earth System processes. This energy flows into the Atmosphere and heats this system up It also heats up the Hydrosphere and the land surface of the Geosphere, and fuels many processes in the Biosphere. Differences in the amount of energy absorbed in different places set the Atmosphere and oceans in motion and help determine their overall temperature and chemical structure. These motions, such as wind patterns and ocean currents redistribute energy throughout the environment. Eventually, the energy that began as Sunshine (short-wave radiation) leaves the planet as Earthshine (light reflected by the Atmosphere and surface back into space) and infrared radiation (heat, also called longwave radiation) emitted by all parts of the planet which reaches the top of the Atmosphere. This flow of energy from the Sun, through the environment, and back into space is a major connection in the Earth system; it defines Earth’s climate.
Biogeochemical Cycles:
There are many ways in which the energy, water, and biogeochemical cycles (cycles of the elements that involve life, chemicals, and the solid Earth) interact and influence the Earth System.
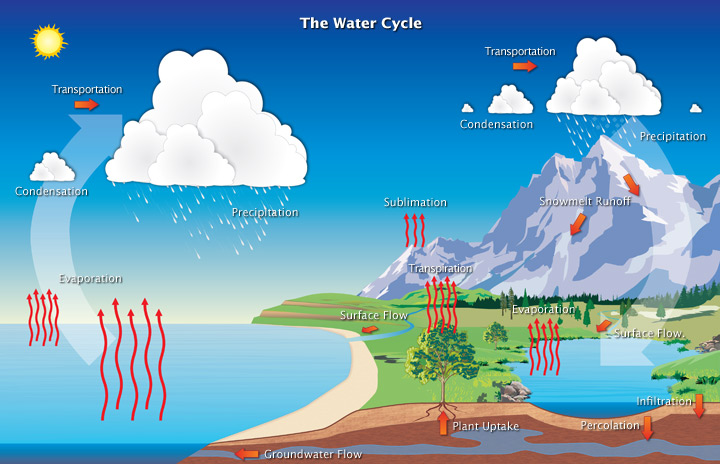
Water Cycle (Hydrologic Cycle)
Water is practically everywhere on Earth. Viewed from space, one of the most striking features of our home planet is the water, in both liquid and frozen forms, that covers approximately 75% of the Earth’s surface. Geologic evidence suggests that large amounts of water have likely flowed on Earth for the past 3.8 billion years—most of its existence. Believed to have initially arrived on Earth’s surface through the emissions of ancient volcanoes, water is a vital substance that sets the Earth apart from the rest of the planets in our solar system. In particular, water appears to be a necessary ingredient for the development and nourishment of life.
Water is the only common substance that can exist naturally as a gas, liquid, or solid at the relatively small range of temperatures and pressures found on the Earth’s surface. Sometimes, all three states are even present in the same time and place, such as this wintertime eruption of a geyser in Yellowstone National Park.
In all, the Earth’s water content is about 1.39 billion cubic kilometers (331 million cubic miles), with the bulk of it, about 96.5%, being in the global oceans. As for the rest, approximately 1.7% is stored in the polar icecaps, glaciers, and permanent snow, and another 1.7% is stored in groundwater, lakes, rivers, streams, and soil. Only a thousandth of 1% of the water on Earth exists as water vapor in the atmosphere.
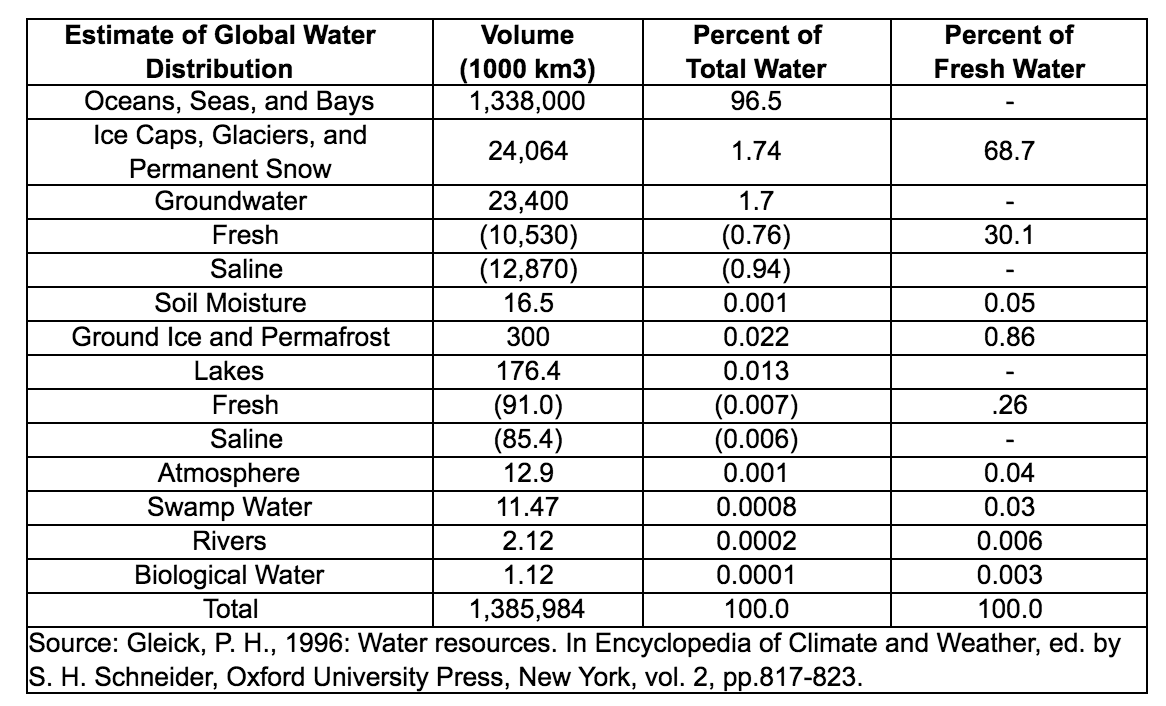
For human needs, the amount of freshwater on Earth—for drinking and agriculture—is particularly important. Freshwater exists in lakes, rivers, groundwater, and frozen as snow and ice. Estimates of groundwater are particularly difficult to make, and they vary widely. (The value in the above table is near the high end of the range.) Groundwater may constitute anywhere from approximately 22 to 30% of fresh water, with ice (including ice caps, glaciers, permanent snow, ground ice, and permafrost) accounting for most of the remaining 78 to 70%.
Groundwater is found in two broadly defined layers of the soil, the “zone of aeration,” where gaps in the soil are filled with both air and water, and, further down, the “zone of saturation,” where the gaps are completely filled with water. The boundary between these two zones is known as the water table, which rises or falls as the amount of groundwater changes.
The amount of water in the atmosphere at any moment in time is only 12,900 cubic kilometers, a minute fraction of Earth’s total water supply: if it were to completely rain out, atmospheric moisture would cover the Earth’s surface to a depth of only 2.5 centimeters. However, far more water—in fact, some 495,000 cubic kilometers of it—are cycled through the Atmosphere every year. It is as if the entire amount of water in the air were removed and replenished nearly 40 times a year.
Despite its small amount, this water vapor has a huge influence on the planet. Water vapor is a powerful greenhouse gas, and it is a major driver of the Earth’s weather and climate as it travels around the globe, transporting latent heat with it. Latent heat is heat obtained by water molecules as they transition from liquid or solid to vapor; the heat is released when the molecules condense from vapor back to liquid or solid form, creating cloud droplets and various forms of precipitation.
Water vapor—and with it energy—is carried around the globe by weather systems. This satellite image shows the distribution of water vapor over Africa and the Atlantic Ocean. White areas have high concentrations of water vapor, while dark regions are relatively dry. The brightest white areas are towering thunderclouds.
Overview
The water, or hydrologic, cycle describes the journey of water as water molecules make their way from the Earth’s surface to the Atmosphere and back again, in some cases to below the surface. This gigantic system, powered by energy from the Sun, is a continuous exchange of moisture between the oceans, the atmosphere, and the land.
Water molecules can take an immense variety of routes and branching trails that lead them again and again through the three phases of ice, liquid water, and water vapor. For instance, the water molecules that once fell 100 years ago as rain on your great- grandparents’ farmhouse in Iowa might now be falling as snow on your driveway in California. Water at the bottom of Lake Superior may eventually rise into the atmosphere and fall as rain in Massachusetts. Runoff from the Massachusetts rain may drain into the Atlantic Ocean and circulate northeastward toward Iceland, destined to become part of a floe of sea ice, or, after evaporation to the atmosphere and precipitation as snow, part of a glacier.
Water continually evaporates, condenses, and precipitates, and on a global basis, evaporation approximately equals precipitation. Because of this equality, the total amount of water vapor in the atmosphere remains approximately the same over time. However, over the continents, precipitation routinely exceeds evaporation, and conversely, over the oceans, evaporation exceeds precipitation.
In the case of the oceans, the continual excess of evaporation versus precipitation would eventually leave the oceans empty if they were not being replenished by additional means. Not only are they being replenished, largely through runoff from the land areas, but over the past 100 years, they have been over-replenished: sea level around the globe has risen approximately 17 centimeters over the course of the twentieth century. The main source of this excess runoff from land contributing to sea level rise is the melting of land ice, particularly in Greenland and Antarctica.
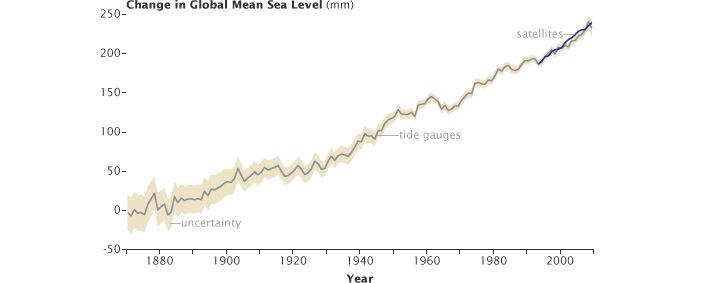
Sea level has risen both because of warming of the oceans, causing water to expand and increase in volume, and because more water has been entering the ocean than the amount leaving it through evaporation or other means. A primary cause for increased mass of water entering the ocean is the calving or melting of land ice (ice sheets and glaciers). Sea ice is already in the ocean, so increases or decreases in the annual amount of sea ice do not significantly affect sea level.

Throughout the hydrologic cycle, there are many paths that a water molecule might follow. Water at the bottom of Lake Superior may eventually rise into the atmosphere and fall as rain in Massachusetts. Runoff from the Massachusetts rain may drain into the Atlantic Ocean and circulate northeastward toward Iceland, destined to become part of a floe of sea ice, or, after evaporation to the atmosphere and precipitation as snow, part of a glacier.
Water molecules can take an immense variety of routes and branching trails that lead them again and again through the three phases of ice, liquid water, and water vapor. For instance, the water molecules that once fell 100 years ago as rain on your great- grandparents’ farmhouse in Iowa might now be falling as snow on your driveway in California.
Evaporation, Transpiration, Sublimation
Together, evaporation, transpiration, and sublimation, plus volcanic emissions, account for almost all the water vapor in the Atmosphere that isn’t inserted through human activities. Studies show that evaporation—the process by which water changes from a liquid to a gas—from oceans, seas, and other bodies of water (lakes, rivers, streams) provides nearly 90% of the moisture in our atmosphere. Most of the remaining 10% found in the atmosphere is released by plants through transpiration where plants take in water through their roots, then release it through small pores on the underside of their leaves. For example, a cornfield 1 acre in size can transpire as much as 4,000 gallons of water every day. In addition, a very small portion of water vapor enters the Atmosphere through sublimation, the process by which water changes directly from a solid (ice or snow) to a gas. The gradual shrinking of snow banks in cases when the temperature remains below freezing results from sublimation.
Condensation & Precipitation
After the water enters the lower atmosphere, rising air currents carry it upward, often high into the atmosphere, where the rising air cools. In cooled air, water vapor is more likely to condense from a gas to a liquid to form cloud droplets. Cloud droplets can grow and produce precipitation (including rain, snow, sleet, freezing rain, and hail), which is the primary mechanism for transporting water from the atmosphere back to the Earth’s surface.
When precipitation falls over the land surface, it follows various routes in its subsequent paths. Some of it evaporates, returning to the atmosphere; some seeps into the ground as soil moisture or groundwater; and some runs off into rivers and streams. Almost all of the water eventually flows into the oceans or other bodies of water, where the cycle continues. At different stages of the cycle, some of the water is intercepted by humans or other life forms for drinking, washing, irrigating, and a large variety of other uses.
Sea Level Rise
Sea level has been rising over the past century, partly due to thermal expansion of the ocean as it warms, causing water to expand and increase in volume, and partly due to the melting of glaciers and ice caps because more water has been entering the ocean than the amount leaving it through evaporation or other means. (Graph ©2010 Australian Commonwealth Scientific and Research Organization.)
Snow and Ice Melt
A primary cause for increased mass of water entering the ocean is the calving or melting of land ice (ice sheets and glaciers). Sea ice is already in the ocean, so increases or decreases in the annual amount of sea ice do not significantly affect sea level.
Credit: NASA Earth Observatory
The Changing Nitrogen Cycle
Credit: UCAR Center for Science Education
Plants and animals could not live without the essential element, nitrogen. It makes up many biological structures and processes such as cells, amino acids, and proteins. It is also necessary for plants to produce chlorophyll, which they use in photosynthesis to make their food and energy.
Nitrogen forms simple chemicals called amino acids, the essential building blocks of proteins and enzymes. It helps plants use carbohydrates to gain energy, like certain foods we eat help us to gain energy. Nitrogen controls how plants take their form and how they function inside, and nitrogen helps plants make the protein that helps them grow strong and healthy. Humans and animals benefit from eating vegetables and plants that are rich in nitrogen because proteins are passed on to humans and animals when they eat vegetables and plants.
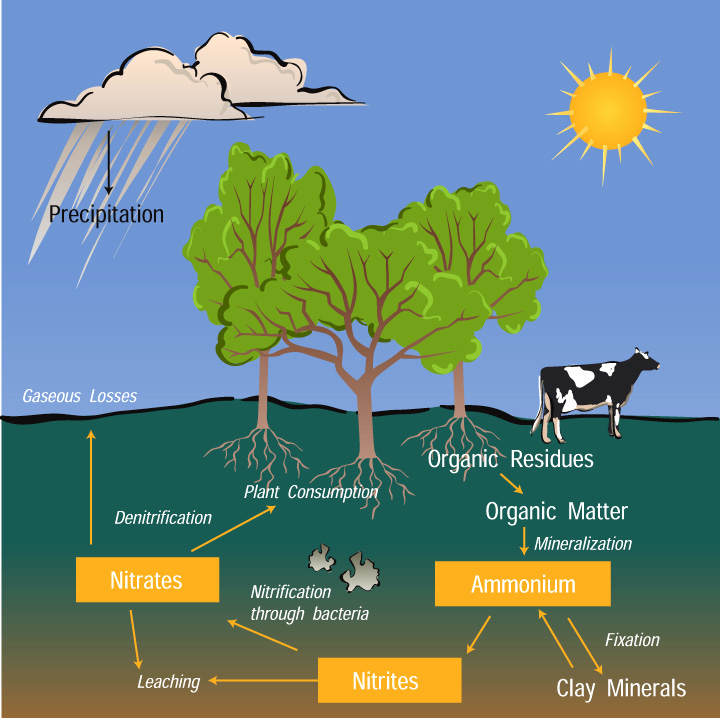
We might commonly think of Earth as having an oxygen-dominated atmosphere, but in reality, the molecule makes up a little less than 20% our air. Most of what surrounds us is nitrogen, at 78 percent in the form of diatomic nitrogen gas, the gas itself is very unreactive. plants and animals simply cannot absorb the gas directly from the atmosphere. Nitrogen, in the forms of Nitrates (NO3), Nitrites (NO2), and Ammonium (NH4), is a nutrient needed for plant growth. Plants take up nitrogen in forms of nitrate ( NO3-) and ammonium ( NH4+ ). Most plants thrive on equal amounts of these ions but nitrates are more quickly available to plants because they move through the soil solution, whereas ammonium ions become fixed or held on to clay particles, called colloids, because of their positive charge.
How Plants Take Up Nitrogen
The nitrogen cycle involves certain processes that change nitrogen into different forms. Unfortunately, these forms of nitrogen are not always used by plants because they either get onto clay particles in soil, they leach into the groundwater because they cannot be absorbed by the soil, or they change into nitrogen gases that escape into Earth's atmosphere. So how does nitrogen change states from N2 in the air to these other states so that they are accessible by the Biosphere? Luckily there are specific kinds of microorganisms living in the soil that can convert gaseous forms of nitrogen into inorganic nitrogen that plants can use.
Specialized bacteria in soil (and certain types of algae in water) can fix nitrogen. These bacteria that cling to roots within the soil convert (or "fix") this inorganic nitrogen into organic forms (ammonia and nitrate ions) that plants can absorb. This process of converting nitrogen to a “biologically available” form - in other words, converting nitrogen gas to a form that plants can use - is referred to as nitrogen fixation. Lightning strikes also result in some nitrogen fixation by splitting the nitrogen molecule into free nitrogen, which immediately reacts with oxygen in the air to form nitrogen oxides. Some of these nitrogen oxide gases dissolve in rainwater and eventually percolate into the soil (Pedosphere). The nutrients needed for plant growth are drawn from the soil from the roots to the leaves. Therefore, any organism (including humans) consuming the nuts, leaves, seeds, roots, tubercles, or fruits of plants can digest this organic form of nitrogen. The Nitrogen Cycle is this process of moving nitrogen among plants, animals, bacteria, the atmosphere, and soil in the ground. This cycle is continuous.
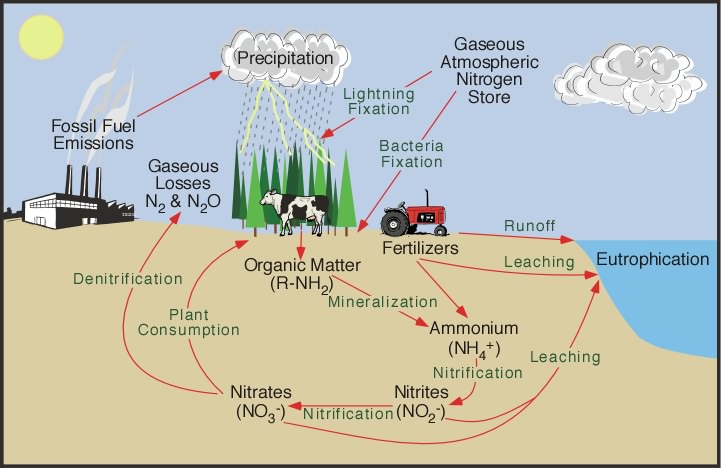
Human activities have a large impact on global nitrogen cycles. In agriculture, soils are generally not rich enough in fixed nitrogen to sustain repetitive crop yields year after year; as a result, farmers use compost heaps or add industrially mass-produced fertilizers such as ammonium nitrate (containing high amounts of organic nitrogen), to enhance the soil.
What Happens When Plants Don't Get Enough Nitrogen:
Plants deficient in nitrogen have thin, spindly stems and their growth is stunted. Their older leaves turn yellowish-green from the lack of chlorophyll produced in the leaves (chlorosis), while newer leaves are supplied with the available nitrogen and sufficient chlorophyll.
What Happens When Plants Get Too Much Nitrogen:
Plants that get too much nitrogen have a lot of foliage (leaf) growth but are not strong. Plants that are not strong can get diseases more easily, can be bothered more by bugs, and can eventually fall over and die. An excess amount of nitrogen in plants can affect the amount of sugar and vitamins in fruits and vegetables, making them taste different. More importantly, excess nitrogen can build up in plant tissues causing toxicity (poisoning) in livestock and in small children who eat nitrogen-rich, leafy vegetables. As we produce synthetic fertilizers, burn fossil fuels, grow legumes such as soybeans as a crop (which fix nitrogen), and clear, burn, and drain wetlands, we release nitrogen in forms that plants use. We have made the amount of biologically available nitrogen through human activity much greater than the nitrogen fixed by bacteria, algae, and lightning.
The Nitrogen Cycle Processes:
- Fixation - Fixation is the first step in the process of making nitrogen usable by plants. Here bacteria change nitrogen into ammonium.
- Nitrification - This is the process by which ammonium gets changed into nitrates by bacteria. Nitrates are what the plants can then absorb.
- Assimilation - This is how plants get nitrogen. They absorb nitrates from the soil into their roots. Then the nitrogen gets used in amino acids, nucleic acids, and chlorophyll.
- Ammonification (or mineralization) - This is part of the decaying process. When a plant or animal dies, decomposers like fungi and bacteria turn the nitrogen back into ammonium so it can reenter the nitrogen cycle.
-
Denitrification - Extra nitrogen in the soil gets put back out into the air. There are special bacteria that perform this task as well.
Climate Program Office - NOAA
Nitrogen As a Pollutant in the Atmosphere
Nitrogen dioxide (NO2) is a gas that occurs naturally in our atmosphere, but in concentrations very low as compared to oxygen (O2) and nitrogen (N2). NO2 is also common pollutant produced primarily during the combustion of gasoline in vehicle engines and coal in power plants. NO2 is part of a family of chemical compounds collectively called “nitrogen oxides” or “NOx”. Nitric oxide (NO) is also part of the NOx family. Together, NO and NO2 play important roles in the chemical formation of ozone near the Earth's surface, as well as contribute to smog when combined with oxygen molecules and the fumes from paint and gasoline (called Volatile Organic Compounds or VOC’s). These compounds also contribute to the production of acid rain when mixed with water vapor forming nitric acid.
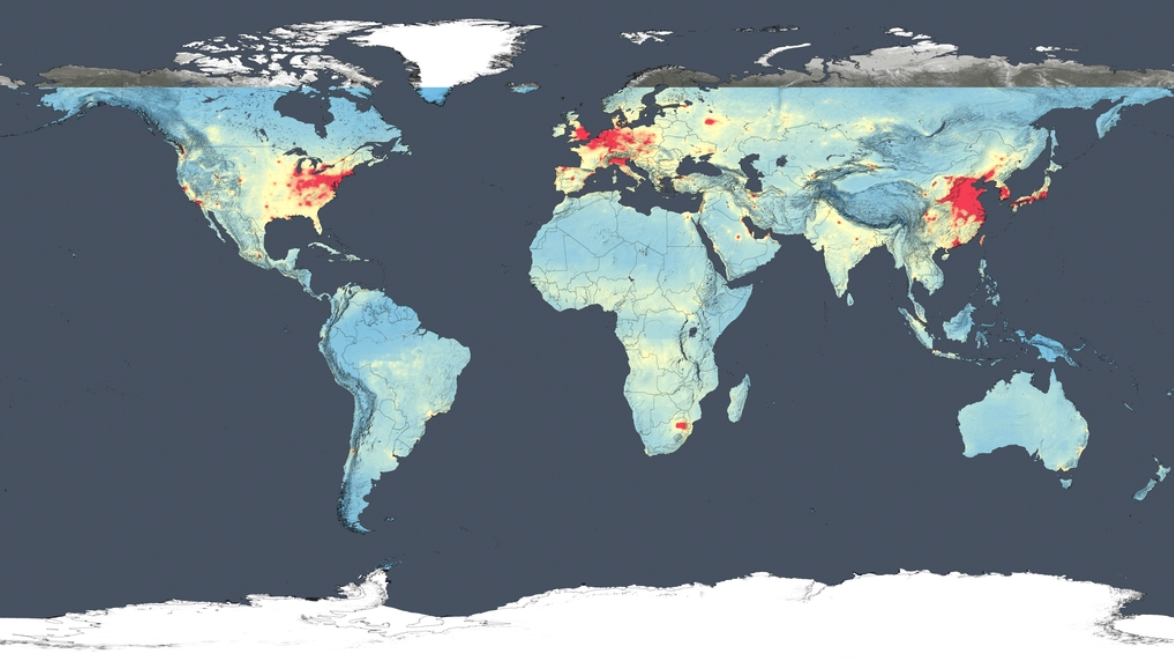
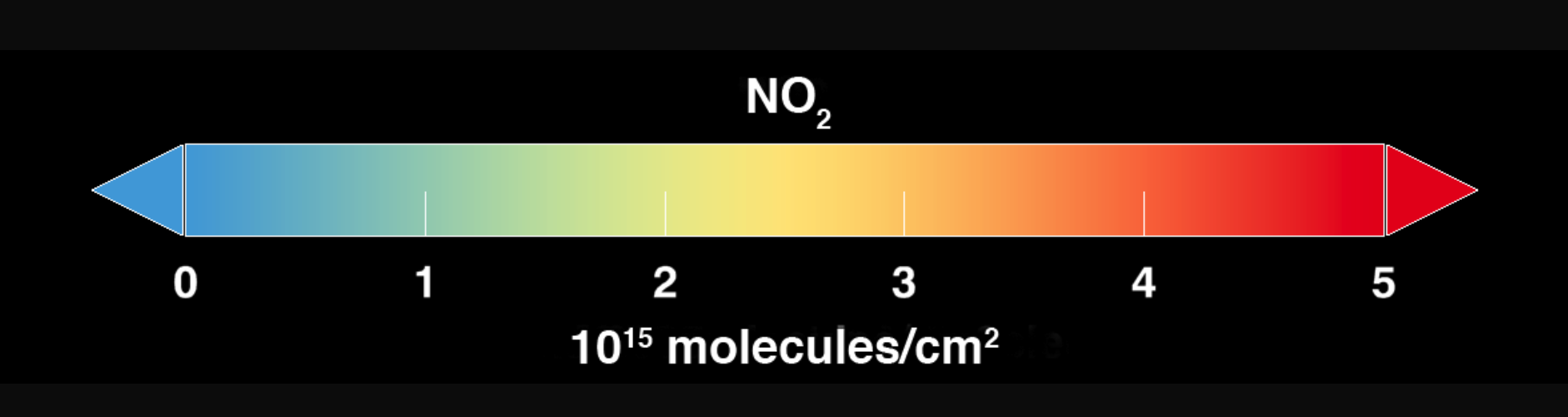
Using new, high-resolution global satellite maps of air quality indicators, NASA scientists tracked air pollution trends over the last decade in various regions and 195 cities around the globe. According to recent NASA research findings, the United States, Europe, and Japan have improved air quality thanks to emission control regulations, while China, India and the Middle East, with their fast-growing economies and expanding industry, have seen more air pollution.
Ozone occurs naturally in the air we breathe, but there's not enough of it to hurt us. Ozone high in the atmosphere (i.e., in the stratospheric “ozone layer”) protects us; it is like sunscreen, protecting us from harmful ultraviolet (UV) rays from the Sun. Near the ground though, ozone is a pollutant. It damages our lungs and harms plants, including the plants we eat. Unhealthy levels of ozone form when there is a lot of NO2 in the air. NO2—and ozone—concentrations are usually highest in cities since NO2 is released into the atmosphere when we burn gas in our cars or coal in our power plants, both things that happen more in cities.
Nitrogen dioxide breaks apart in sunlight releasing free oxygen atoms to connect onto oxygen molecules forming dangerous ground-level ozone. Since sunlight is an important ingredient in the formation of high concentrations of ozone, ozone in urban areas tends to be greatest in summer when sunlight is strongest. NO2 is also unhealthy to breathe in high concentrations, such as on busy streets and highways where there are lots of cars and trucks. When driving, it is typically a good idea to keep the car windows rolled up and the car's ventilation set to “recirculate” so as to keep pollution out of the interior of the car. It is also important to reduce outdoor activities like playing or jogging if government officials warn you that air quality will be bad on a certain day.
Nitrous oxide (N20) is a powerful greenhouse gas, which traps heat near the Earth’s surface. You may have heard of it before, referred to as “laughing gas” which is a common medical treatment to decrease pain. It is produced almost entirely at the Earth's surface, about 70% from through natural processes in the Earth’s Biosphere (tiny microbes that alter nitrogen in the soils of tropical forests and in the oceans) and the rest from human activities (e.g. from farm animals, sewage, and fertilizers, as well as fossil-fuel burning). Quantities of nitrous oxide have increased since the Industrial Revolution in the Atmosphere as Earth’s climate has gotten warmer. scientists have observed an increase in N2O of about 0.3%/year since the 1950's.
For more information about the Nitrogen Cycle, visit UCAR.
The Carbon Cycle
Credit: NASA Earth Observatory

Carbon is both the foundation of all life on Earth, and the source of the majority of energy consumed by human civilization. [Photographs ©2007 MorBCN (top) and ©2009 sarahluv (lower).]
Carbon is a fundamental part of the Earth system. It is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. Forged in the heart of aging stars, carbon is the fourth most abundant element in the Universe. Most of Earth’s carbon—about 65,500 billion metric tons—is stored in rocks. The rest is in the ocean, atmosphere, plants, soil, and fossil fuels. Carbon moves from the atmosphere to the land, ocean, and life through biological, chemical, geological and physical processes in a cycle called the carbon cycle. Any change in the cycle that shifts carbon out of one reservoir puts more carbon in the other reservoirs. Carbon flows between each reservoir in slow and fast cycles.
This diagram of the fast carbon cycle shows the movement of carbon between land, atmosphere, and oceans. Yellow numbers are natural fluxes, and red are human contributions in gigatons of carbon per year. White numbers indicate stored carbon.
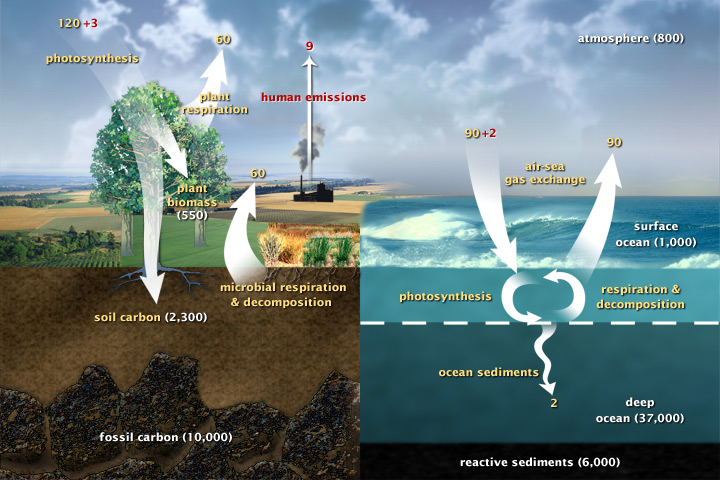
Over the long term, the carbon cycle seems to maintain a balance that prevents all of Earth’s carbon from entering the atmosphere (as is the case on Venus) or from being stored entirely in rocks. This balance helps keep Earth’s temperature relatively stable, like a thermostat. This thermostat works over a few hundred thousand years, as part of the slow carbon cycle. This means that for shorter time periods—tens to a hundred thousand years—the temperature of Earth can vary. And, in fact, Earth swings between ice ages and warmer interglacial periods on these time scales. Parts of the carbon cycle may even amplify these short-term temperature changes.

On very long time scales (millions to tens of millions of years), the movement of tectonic plates and changes in the rate at which carbon seeps from the Earth’s interior may change the temperature on Earth’s thermostat. Earth has undergone such a change over the last 50 million years, from the extremely warm climates of the Cretaceous (roughly 145 to 65 million years ago) to the glacial climates of the Pleistocene (roughly 1.8 million to 11,500 years ago). [See Divisions of Geologic Time—Major Chronostratigraphic and Geochronologic Units for more information about geological eras.]
Slow Cycle
Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 million years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, 1013 to 1014 grams (10–100 million metric tons) of carbon move through the slow carbon cycle every year. In comparison, human emissions of carbon to the atmosphere are on the order of 1015 grams (1 Billion Metric Tons), whereas the fast carbon cycle moves 1016 to 1017 grams (10-100 billion Billion Metric Tons) of carbon per year.
The movement of carbon from the Atmosphere to the Geosphere (rocks) begins with rain. Atmospheric carbon combines with water to form a weak acid—carbonic acid—that falls to the surface in rain. The acid dissolves rocks—a process called chemical weathering—and releases calcium, magnesium, potassium, or sodium ions. Rivers carry the ions to the ocean. Rivers carry calcium ions—the result of chemical weathering of rocks—into the ocean, where they react with carbonate dissolved in the water. The product of that reaction, calcium carbonate, is then deposited onto the ocean floor, where it becomes limestone. In the ocean, the calcium ions combine with bicarbonate ions to form calcium carbonate, the active ingredient in antacids and the chalky white substance that dries on your faucet if you live in an area with hard water. In the modern ocean, most of the calcium carbonate is made by shell-building (calcifying) organisms (such as corals) and plankton (like coccolithophores and foraminifera). After the organisms die, they sink to the seafloor. Over time, layers of shells and sediment are cemented together and turn to rock, storing the carbon in stone—limestone and its derivatives. Limestone, or its metamorphic cousin, marble, is rock made primarily of calcium carbonate. These rock types are often formed from the bodies of marine plants and animals, and their shells and skeletons can be preserved as fossils. Carbon locked up in limestone can be stored for millions—or even hundreds of millions—of years. Only 80 percent of carbon-containing rock is currently made this way. The remaining 20 percent contain carbon from living things (organic carbon) that have been embedded in layers of mud. Heat and pressure compress the mud and carbon over millions of years, forming sedimentary rock such as shale. In special cases, when dead plant matter builds up faster than it can decay, layers of organic carbon become oil, coal, or natural gas instead of sedimentary rock like shale. This coal seam in Scotland was originally a layer of sediment, rich in organic carbon. The sedimentary layer was eventually buried deep underground, and the heat and pressure transformed it into coal. Coal and other fossil fuels are a convenient source of energy, but when they are burned, the stored carbon is released into the atmosphere. This alters the balance of the carbon cycle and is changing Earth’s climate.

The slow cycle returns carbon to the atmosphere through volcanoes. Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. The heated rock recombines into silicate minerals, releasing carbon dioxide. When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year—100–300 times more than volcanoes—by burning fossil fuels.
Chemistry regulates this dance between ocean, land, and atmosphere. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering. Carbon stored in rocks is naturally returned to the atmosphere by volcanoes. In this photograph, Russia’s Kizimen Volcano vents ash and volcanic gases in January 2011. Kizimen is located on the Kamchatka Peninsula, where the Pacific Plate is subducting beneath Asia. However, the slow carbon cycle also contains a slightly faster component: the ocean. At the surface, where air meets water, carbon dioxide gas dissolves in and ventilates out of the ocean in a steady exchange with the atmosphere. Once in the ocean, carbon dioxide gas reacts with water molecules to release hydrogen ions, making the ocean more acidic. The hydrogen reacts with carbonate from rock weathering to produce bicarbonate ions.
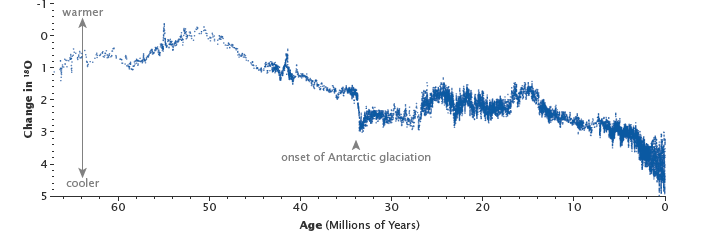
Before the industrial age, the ocean vented carbon dioxide to the atmosphere in balance with the carbon the ocean received during rock weathering. However, since carbon concentrations in the atmosphere have increased, the ocean now takes more carbon from the atmosphere than it releases. Over millennia, the ocean will absorb up to 85 percent of the extra carbon people have put into the atmosphere by burning fossil fuels, but the process is slow because it is tied to the movement of water from the ocean’s surface to its depths.In the meantime, winds, currents, and temperature control the rate at which the ocean takes carbon dioxide from the atmosphere. (See The Ocean’s Carbon Balance on the Earth Observatory.) It is likely that changes in ocean temperatures and currents helped remove carbon from and then restore carbon to the atmosphere over the few thousand years in which the ice ages began and ended. This means that for shorter time periods—tens to a hundred thousand years—the temperature of Earth can vary. And, in fact, Earth swings between ice ages and warmer interglacial periods on these time scales. Parts of the carbon cycle may even amplify these short-term temperature changes.The uplift of the Himalaya, beginning 50 million years ago, reset Earth’s thermostat by providing a large source of fresh rock to pull more carbon into the slow carbon cycle through chemical weathering. The resulting drop in temperatures and the formation of ice sheets changed the ratio between heavy and light oxygen in the deep ocean, as shown in this graph. (Graph based on data from Zachos at al., 2001.)

Fast Cycle
The time it takes carbon to move through the fast carbon cycle is measured in a lifespan, which may not seem very quick. The fast carbon cycle is largely the movement of carbon through life forms on Earth or the Biosphere. Between 1015 and 1017 grams (1,000 to 100,000 million metric tons) of carbon move through the fast carbon cycle every year. On this time scale, the carbon cycle is most visible in life. Carbon plays an essential role in biology because of its ability to form many bonds—up to four per atom—in a seemingly endless variety of complex organic molecules. Plants and phytoplankton (microscopic organisms in the ocean) are the main components of the fast carbon cycle and convert carbon dioxide to biomass (like leaves and stems) through photosynthesis. In this process, they take carbon dioxide (CO2) from the atmosphere by absorbing it into their cells with water to form sugar (CH2O) and oxygen. The chemical reaction looks like this:
CO2 + H2O + energy = CH2O + O2
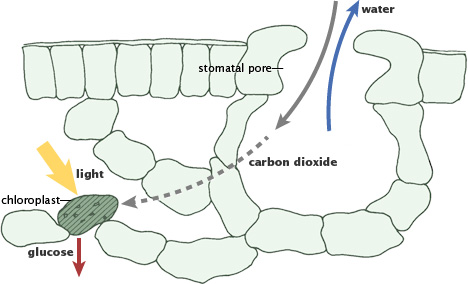
During photosynthesis, plants absorb carbon dioxide and sunlight to create fuel—glucose and other sugars—for building plant structures. This process forms the foundation of the fast (biological) carbon cycle. (Illustration adapted from P.J. Sellers et al., 1992.)
During photosynthesis, plants absorb carbon dioxide and sunlight to create fuel—glucose and other sugars—for building plant structures. This process forms the foundation of the fast (biological) carbon cycle. (Illustration adapted from P.J. Sellers et al., 1992.)The bonds in the long carbon chains contain a lot of energy. When the chains break apart, the stored energy is released. This energy makes carbon molecules an excellent source of fuel for all living things. The carbon returns to the Atmosphere in the following processes but all involve the same chemical reaction:
- when plants and phytoplankton break down the sugar to get the energy they need to grow
- when plants and phytoplankton die or decay (and are eaten by bacteria) at the end of the growing season
- when plants and phytoplankton are eaten and digested by animals (including people) to get energy
- when plants and phytoplankton burn in fires
In each case, oxygen combines with sugar to release water, carbon dioxide, and energy. The basic chemical reaction looks like this:
CH2O + O2 = CO2 + H2O + energy
These four processes move carbon from a plant and put carbon gases into the Atmosphere. Changes that return carbon to the Atmosphere result in warmer temperatures on Earth.
The fast carbon cycle is so tightly tied to plant life that the growing season can be seen by the way carbon dioxide fluctuates in the atmosphere. In the Northern Hemisphere winter, when few land plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. During the spring, when plants begin growing again, concentrations drop. It is as if the Earth is breathing. Because plants and animals are an integral part of the carbon cycle, the carbon cycle is closely connected to ecosystems. As ecosystems change under a changing climate, the carbon cycle will also change. For example, plants may bloom earlier in the year and grow for more months (assuming sufficient water is present) as the growing season gets longer, altering the food supply for animals in the ecosystem. If more plants grow, they will take more carbon out of the atmosphere and cool temperatures. If, on the other hand, warming slows plant growth, habitats will shift and more carbon will go into the atmosphere where it can cause additional warming.
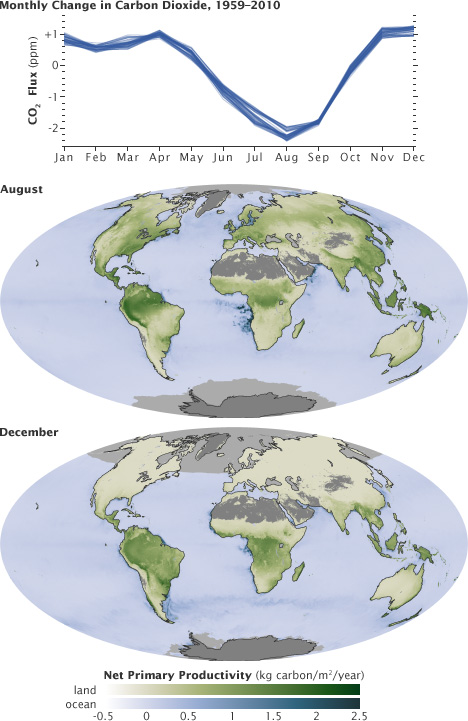
This cycle peaks in August, with about 2 parts per million of carbon dioxide drawn out of the atmosphere. In the fall and winter, as vegetation dies back in the northern hemisphere, decomposition and respiration return carbon dioxide to the atmosphere.
These maps show net primary productivity (the amount of carbon consumed by plants) on land (green) and in the oceans (blue) during August and December 2010. In August, the green areas of North America, Europe, and Asia represent plants using carbon from the atmosphere to grow. In December, net primary productivity at high latitudes is negative, which outweighs the seasonal increase in vegetation in the southern hemisphere. As a result, the amount of carbon dioxide in the atmosphere increases.
Disciplinary Core Ideas:
Crosscutting Concepts:
- Systems and System Models
- Structure and Function
- Stability and Change